Simultaneous removal of SO2 and production of sulphuric acid using hydrogen peroxide
Abstract
During the extraction of metals from sulphide ores, sulphur dioxide (SO2) is produced. In order to meet emissions standards at an extraction facility, two Macrotek Model MP Packed Bed Scrubbers were installed in series. The scrubber uses hydrogen peroxide (H2O2) to convert SO2 to 40% sulphuric acid (H2SO4) which can be used in the process or sold as a product. The optimized scrubber design minimized supply and installation costs; minimized maintenance; reduced and optimized operating costs; and provided for maximum flexibility, while achieving a removal efficiency well above 99.7%.
Introduction
Roasting is a process where metals are extracted from sulphide ores. During this process, SO2, among other contaminants is produced. SO2 emissions must be controlled due to the health and environmental risks that the gas presents.
Problem
SO2 removal can be accomplished by various processes, most commonly by reaction with an alkaline reagent and forced oxidation with air. Although high removal efficiencies can be achieved, large volumes of by-products are produced as waste. Furthermore, operating issues may occur when systems are scaled down.
Solution
Flue gas desulphurization (FGD) by scrubbing with hydrogen peroxide to produce concentrated sulphuric acid in a packed tower accomplishes many of the objectives set out by Macrotek and the customer. Firstly, as sulphuric acid is required in for acid leaching in other areas of the facility, no waste is generated. Hence, there is no need for costly wastewater treatment or disposal. Secondly, excess sulphuric acid can be sold as a product which makes this process economically favourable. Therefore, this process is particularly attractive when SO2 removal and H2SO4 consumption is required in the plant.

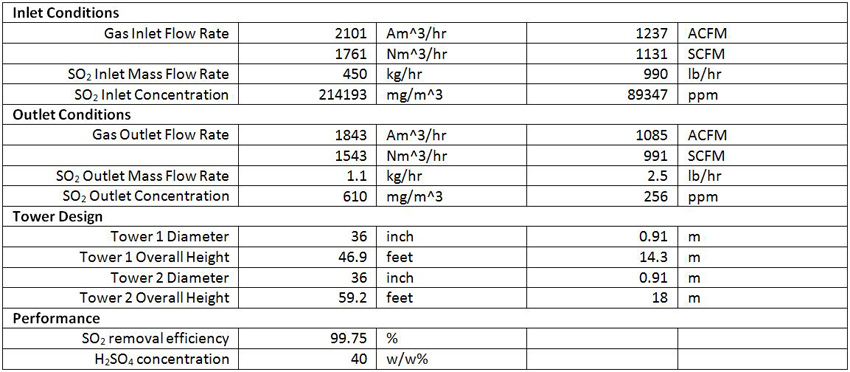
The untreated gas enters the peroxide system for absorption and conversion of SO2 to sulfuric acid as shown in Equation 1 and Equation 2. Two Macrotek Model MP Packed Bed Scrubbers are connected in series in the peroxide system. The gas and recirculating liquid flow counter-currently through the scrubbers as can be seen below. The conversion of SO2 to H2SO4 in Equation 2 is highly exothermic; therefore the recirculating liquid is cooled using a heat exchanger before re-entering the scrubber. H2O2 is continuously added to maintain the required emissions.
Performance
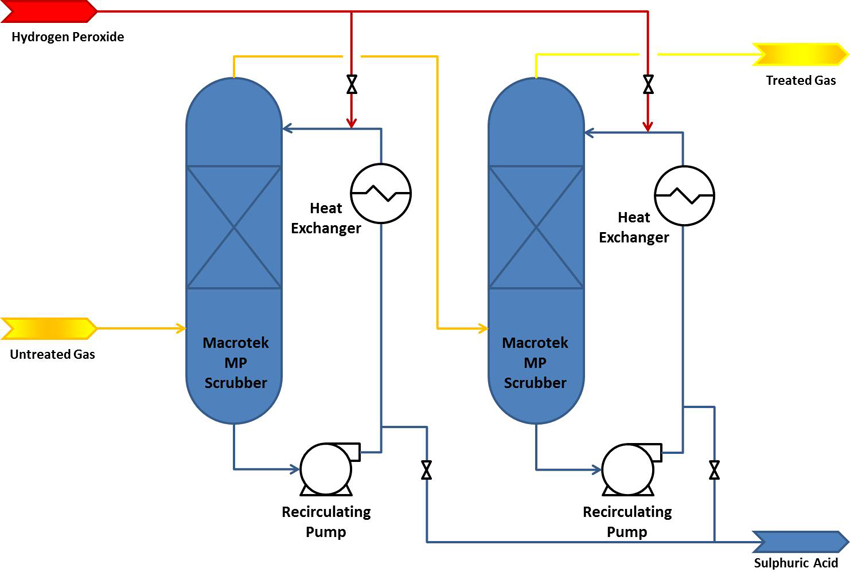
After several years in operation, the SO2 scrubbers are continuously producing 40-46% sulphuric acid and the customer is selling the product. Further, a SO2 removal efficiency of well above 99.7% is being achieved. Process design, tower dimensions and performance is summarized in the table below.